Abstract
Waldenström's macroglobulinemia (WM) is an uncommon lymphoproliferative disorder characterized by monoclonal immunoglobulin M protein production and bone marrow infiltration by lymphoplasmacytoid cells. It remains an incurable disease. IgM monoclonal gammopathy of undetermined significance (MGUS) is the premalignant condition and is associated with the risk of developing WM. MYD88L265P is a recurrent mutation found in more than 90% of WM patients, and this genetic event is detectable in at least half of IgM MGUS patients. However, the precise cellular landscape and the mechanisms of progression from IgM MGUS to WM remain unclear. Single-cell RNA sequencing (scRNA-seq) provides us a powerful approach to explore tumor heterogeneity and identify rare malignant cells. Anti-CD38 monoclonal antibodies have been introduced into the therapeutic arsenal for plasma cell diseases. Therefore, we analyzed CD38+ immune microenvironment together with B cells and plasma cells to illustrate the first cellular landscape of IgM MGUS and WM in single cell resolution.
We isolated bone marrow CD19+ and CD19-CD38+ cells from WM patients(n=3), IgM MGUS patients(n=3) and healthy donors(n=3) and employed the 10x Genomics platform to perform single-cell transcriptomic sequencing. After quality control, our dataset contained a total of 73024 cells. We used UMAP to visualize the cell superclusters and identified clusters including B cells, plasma cells, CD3+CD20+ (CD19+) cells, T cells and NK cells based on the expression of canonical lineage markers and cluster-specific markers. We found that healthy donors had the highest percentage of B cells (72.7% in healthy donors, 19.0% in IgM MGUS patients, 60.5% in WM patients; p<0.001) and that IgM MGUS and WM patients had more NK and T cells than healthy controls (0.06% and 17.7%, respectively, in healthy donors; 24.1% and 20.5% in IgM MGUS patients; 8.5% and 24.0% in WM patients; p<0.001). We also noticed that CD3+CD20+(CD19+) cells were present in both IgM MGUS and WM patients.
First, we investigated the cell clusters correlated with WM pathology including B cells and plasma cells. We compared the B-cell gene expression profiles after removing the early B cells. Compared with those of IgM MGUS patients, mature B cells of WM patients showed upregulation of PIM1, DUSP22, HES1, NEAT1, RGS1, RGS16, and GADD45B. Light chain restriction and higher CNV levels were found in B cells of WM patients. SCENIC analysis showed that FOS, FOSB, EGR1 and JUNB were downregulated in B cells from WM and IgM MGUS patients. REL, a proto-oncogene promoting the survival and proliferation of B lymphocytes, was upregulated in B cells of WM patients, and IRF8 and ELF1 were upregulated in B cells of IgM MGUS patients. Nevertheless, plasma cells of WM patients, IgM MGUS patients and healthy donors were colocalized in one cluster, which might indicate their similar gene expression patterns.
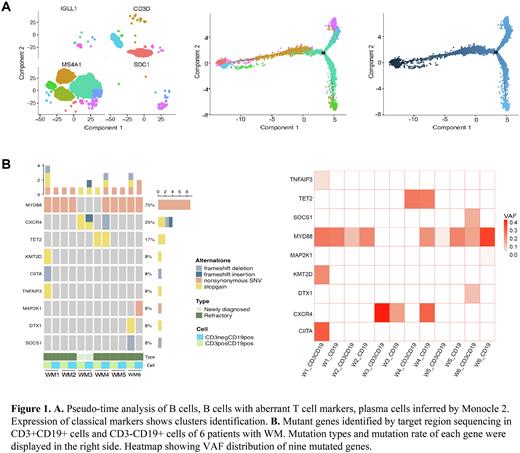
Next, we further determined the characteristics of B-cell subpopulation with aberrant T-cell antigen expression. Trajectory analysis revealed that CD3+CD20+(CD19+) cells and pro-B cells were located at the same end of the branched structure and were then directed toward mature B cell and plasma cell fates, respectively (Figure. 1A). This inferred developmental trajectory suggested that CD3+CD20+(CD19+) cells may be tumor stem cell-like subset. Using flow cytometry, we validated that CD3 was expressed in a fraction of CD19+ cells (mean: 3.69%, range: 0.28%-15.10%) of bone marrow samples from 8 WM patients. In addition, targeted gene sequencing was performed on CD3+CD19+ and CD3-CD19+ cells from 6 WM patients. MYD88 were proved to be the most frequently mutated gene in this cell subset. And we inferred that MYD88 mutation might be the early events in tumorigenesis by VAF analysis (Figure. 1B). Additional subclonal hits, such as CXCR4 and MAP2K1 mutations, could be acquired during tumor progression.
In conclusion, our study provides comprehensive insights into mechanisms of progression from IgM MGUS to WM. We identified the rare CD19+ cell subpopulation with T-cell antigen expression in WM patients. It will be interesting to explore novel therapeutic strategies targeting rare potential cancer-initiating cells in future.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal